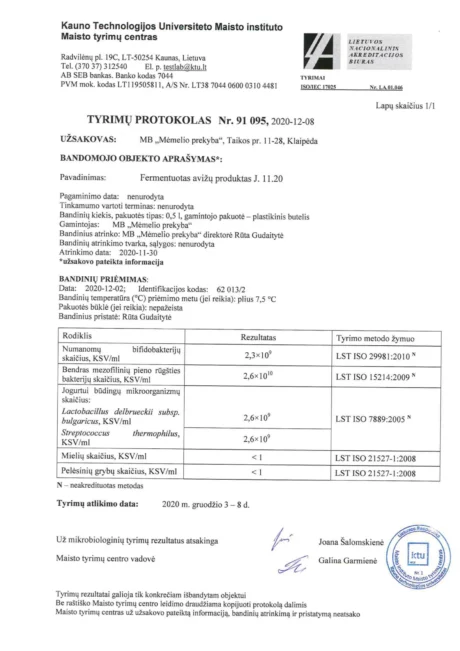
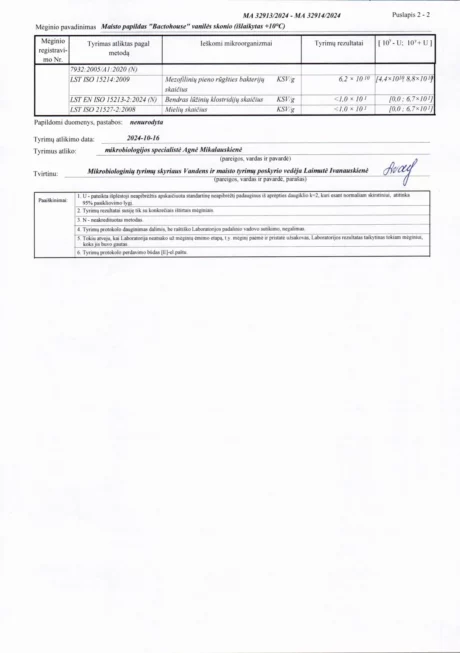
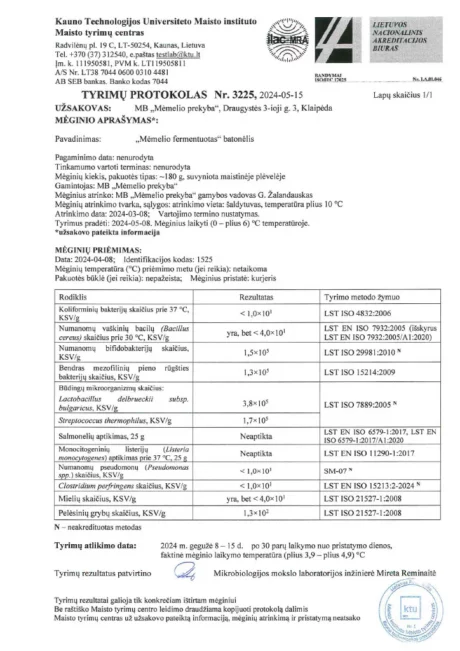
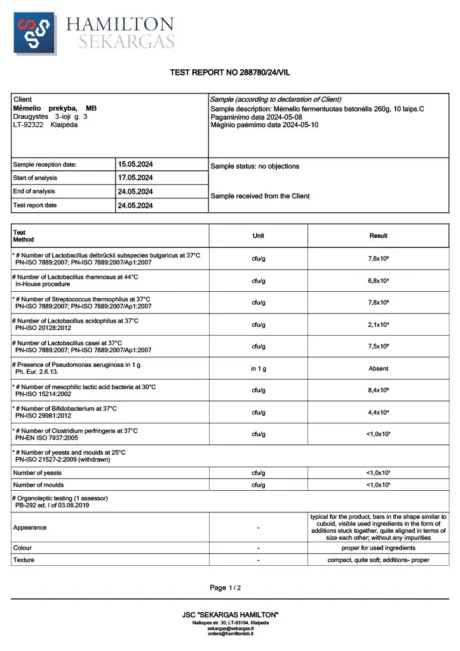
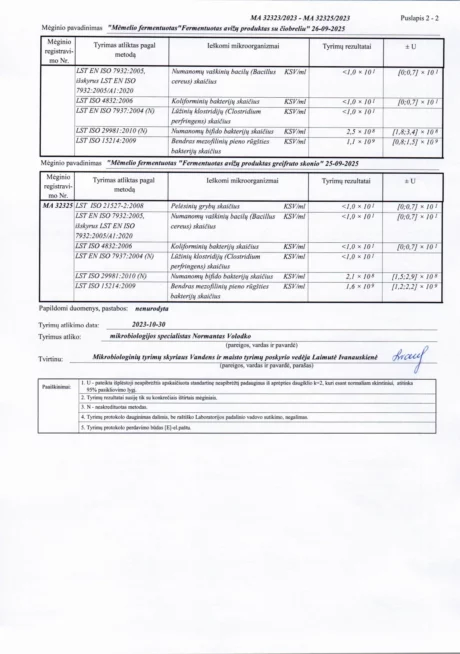
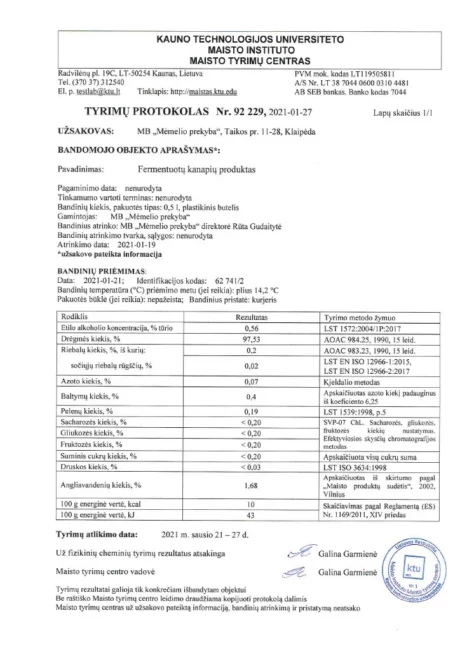
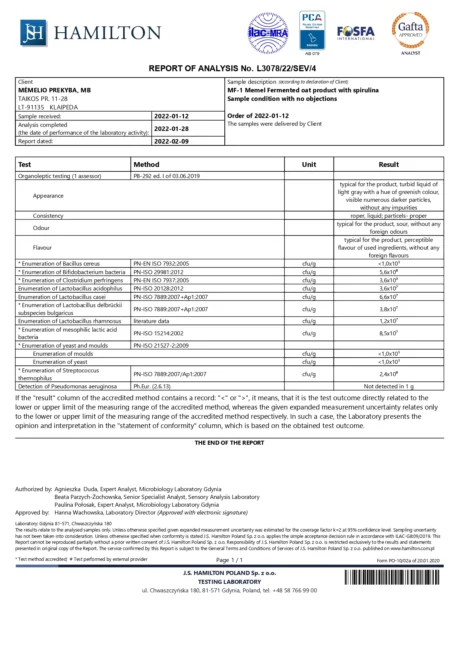
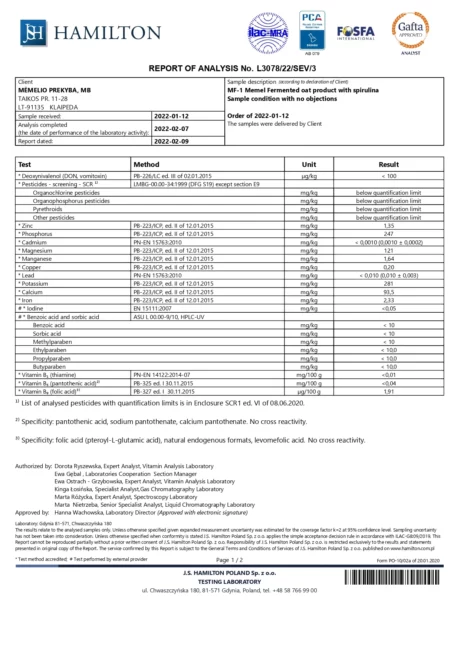
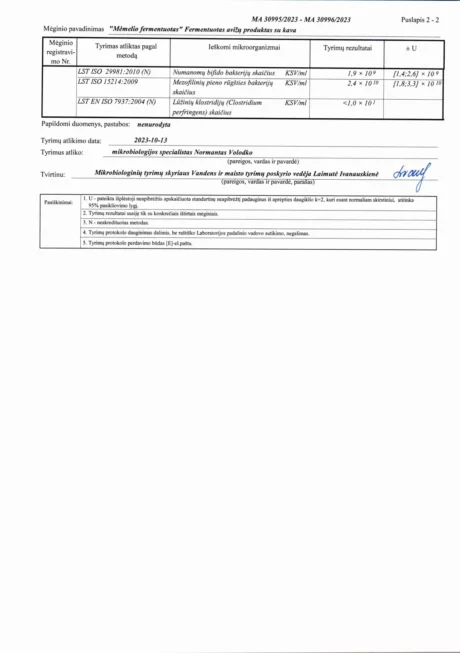
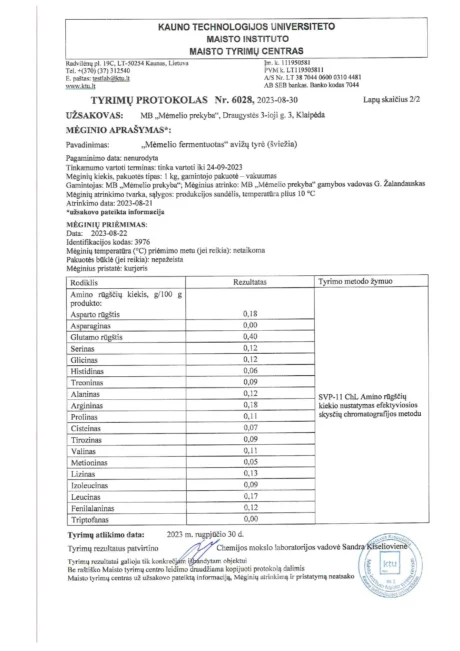
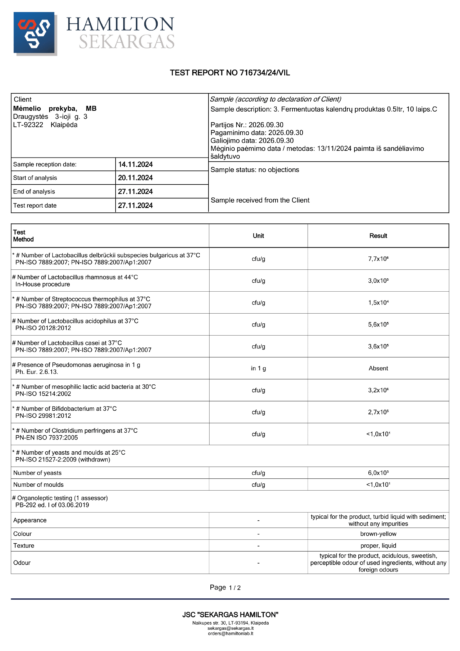
BACTOHOUSE products are regularly tested at the National Public Health Laboratory, the KTU Food Research Centre, Klaipėda University, the National Institute for Food and Veterinary Risk Assessment, the Hamilton Laboratory in Poland and the Sequench Laboratory in New Zealand. The actual content of good bacteria is even higher than declared on the label. 1×10⁹ CFU means one billion colony-forming units, while 1×10¹⁰ means ten billion! And that’s just in one millilitre of the product – imagine how many are in the whole bottle!
Our fermented products contain the following viable bacteria: Lactobacillus delbrückii subsp. Bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium animalis, Bifidobacterium thermophilum, Propionibacterium freudenreichii, Lactobacillus fermentum, Lactobacillus mucosae, Lactobacillus paracasei, Lactococcus lactis.
From 2022, genetic testing by metogenomic sequencing will be carried out periodically in the US by Zymo Research Corporation. You can view the results of the study HERE. This test identifies the complete microbiome, i.e. all microorganisms living in the product, including:
– Detection of all live bacteria (good and bad, if any);
– detection and identification of even dead cells;
– searching for viruses;
– sequencing for mutated bacteria;
– what genera and how many families of bacteria there are in total.
In this way, we can ensure product safety, stability and quality through periodic self-monitoring.
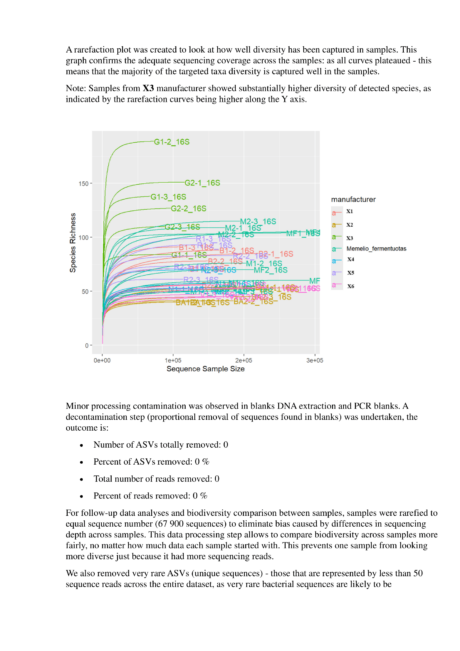
In 2025, a comprehensive sequencing study was carried out by the Sequench laboratory, the report of which can be found here.